The challenges, the pitfalls and how we make use of it all?
In the long-term fight against the spread of SARS-CoV-2, a second round of testing tools has been pushed to the front of the media coverage- so called ‘Serological Testing’. There has been confusion as to what these tests are, and what long-term value they can provide to this now Global pandemic. So how do we disseminate this information and determine what makes a valid serological test for SARS-CoV-2, and exactly what do we do with this information?
What is a serological test for SARS-CoV-2?
The basis of a serological test is the detection of antibodies in the blood, that specifically target segments of the SARS-CoV-2 virus, called antigens. These are regions of the virus, usually proteins, that our bodies detect as foreign and induce an immune response against. There are many different facets of our immune response to infections such as COVID-19, but serological detection focuses on the humoral response and the generation of antibodies: initially IgM, followed by a secondary phase of highly specific IgG antibodies. These immunoglobulins are generated to bind and ultimately neutralize the virus (Fig. 1).
How do serological tests detect SARS-CoV-2 antibodies?
Since SARS-CoV-2 is a completely novel virus, none of us are expected to have circulating antibodies targeting the viral antigens, unless we have been exposed to it, i.e. unless we have been infected. With so many individuals who have not displayed symptoms but who have then tested positive, the spread of the virus has been relentless in areas of high population density, and serological testing will help identify previously exposed individuals.
Alternatively, there are regions of low infection and therefore low population immunity that might be highly susceptible for a second wave in SARS-CoV-2 in the future; knowledge of these areas will enable healthcare systems to better allocate resources to manage future outbreaks.
To date, the limited number of serological studies completed, all suggest that coronavirus infections greatly outnumber RT-PCR confirmed SARS-CoV-2 infections, by a factor of 10 or more (Washington Post, April 28th 2020).
Serological testing in South Korea and Sweden
The scientific community is now looking to assess the various global strategies that have been adopted to combat this pandemic. South Korea’s early focus on large scale RT-PCR and Serological screening appears to have markedly impacted the spread of the infection in that region.
Sweden’s actions towards this pandemic are widely considered to be the most radical, with a focus on establishing ‘herd immunity’ within the country’s population. In contrast to most countries who introduced social distancing and wide-spread quarantine to ‘flatten the curve’, Sweden’s schools, restaurants and business remain open, essentially with a ‘business as usual’ approach.
Although Sweden’s infection rates are slightly higher than their immediate neighbors, both the number of confirmed cases and deaths from SARS-CoV-2 in Sweden have plateaued since the first week of April 2020 (www.covid19insweden.com).
Future serological screening of the Swedish population will provide valuable insights into the long-term outcome of this approach to establish accelerated herd immunity against SARS-CoV-2.
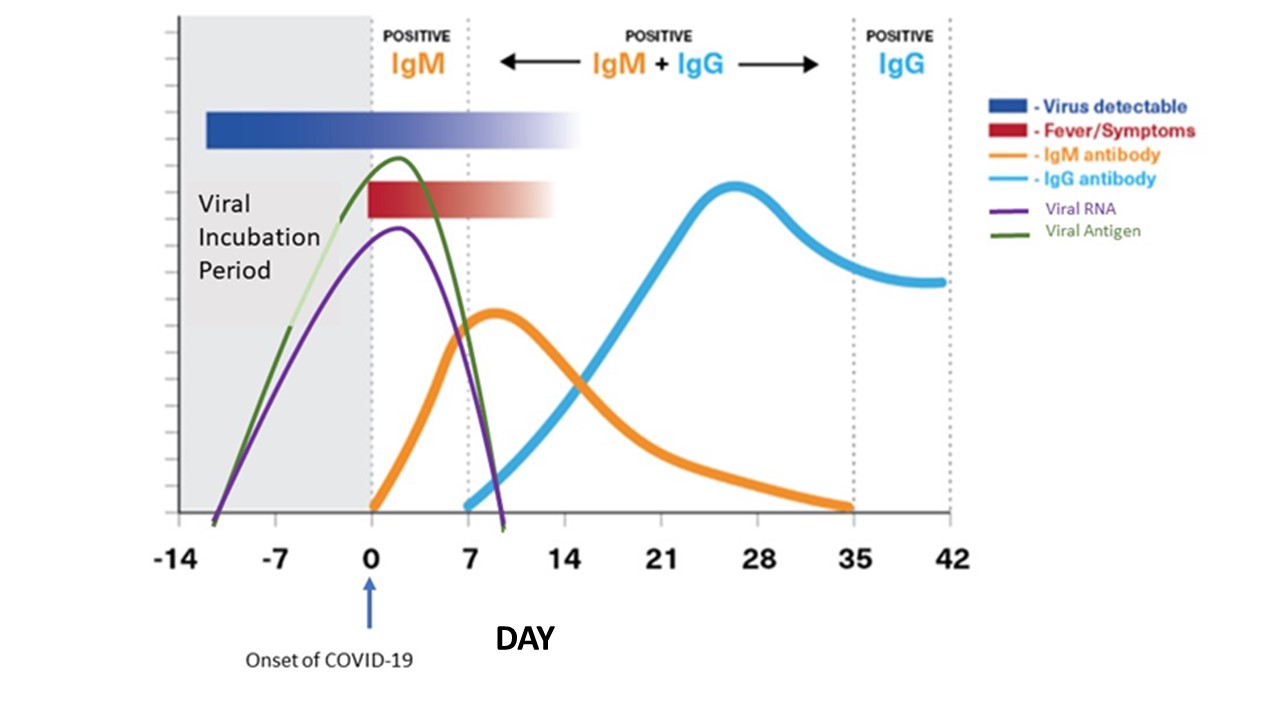
Fig. 1. Typical immune response to coronavirus infection. The viral load increases in the infected individual before the onset of symptoms. The individual may, unknowingly, be able to actively spread the infection during this phase.
As the immune system recognizes the infection, IgM is generated against the virus. This is followed by a second response leading to the production of higher affinity IgG molecules targeting the SARS-CoV-2 virus.
Two ways Serological Testing for Covid-19 Helps Us Now and Into the Future
1. To determine how many people were previously infected with COVID-19
Essentially, serological testing can help healthcare professionals identify individuals who have overcome COVID-19 in the past, and who have developed an immune response to the virus. In combination with other clinical data, this information might be useful in determining individuals who are no longer susceptible to infection and can safely return to work.
The caveat is that no one really knows how long this immunity might last, because immunity functions in a continuum. Infection by pathogens such as the virus causing chicken pox (varicella-zoster virus) can result in near universal, life-long resistance, compared with infection by the tetanus-causing bacteria Clostridium tetani that results in almost no immunological protection. Typical immunity to seasonal coronaviruses (such as those that cause the common cold) starts to decline within weeks of infection.
Our only reasonable insights come from individuals who were infected with the related SARS1 virus during the 2002-3 outbreaks; studies indicated that SARS1-specific antibodies were maintained for an average of 2 years, with significant reduction in antibody levels (or titers) in the third year. The conclusion from this study, was that SARS1 patients might be susceptible to reinfections ≥3 years after their initial exposure (Wu, L-P. et. al. 2007).
2. To source donor serum for Convalescent Plasma treatment
A second, and more immediate need for Serological Testing is in the area of screening convalescent donor serum for anti-SARS-CoV-2 Neutralizing Antibodies- one of the most potent treatment options we currently have against SARS-Cov-2.
As previously discussed, infected patients who have recovered appear to retain circulating antibodies against the SARS-CoV-2 virus for some time. Transfusing the serum containing these antibodies into patients has the potential to help them fend off their own infection by kick starting their own immune system response. This old solution now made new, is known as Convalescent Plasma (or serum) Therapy. It has most recently been used to treat patients with Ebola, SARS, MERS and measles, and was considered a breakthrough in managing the 1918 Pandemic Flu.
There are obvious advantages to this approach, it is readily available from the many recovered SARS-CoV-2 donors, and patients can receive the treatment within 36 hours after collection. But there are many unanswered questions around its adoption, and although the FDA has loosened restrictions around its use, it can still only be used within a clinical trial protocol, or in cases of compassionate use, where no other treatment options exist.
First and foremost though, all donors must meet certain criteria: including being free of hepatitis, not to have traveled outside of the USA within the last year, and have to had been confirmed SARS-CoV-2 positive and subsequently negative twice by the RT-PCR test; with many individuals unable to get tested, many potential donors find themselves ineligible for donation.
But the success of this approach relies on accurate serological screening of the levels of anti-SARS-CoV-2 antibodies in the donor serum, and especially for those potent SARS-CoV-2 Neutralizing Antibodies.
Two Types of Serological Testing Platforms
There are two common platforms for serological testing: Lateral Flow and Enzyme-linked Immunosorbent Assay (ELISA).
1. Lateral flow serological testing
Lateral Flow constitutes a small plastic device that provides a simple and inexpensive platform for the detection of antibodies that cross react with a SARS-CoV-2 antigen. The IgG/IgM test cassette is a membrane-based immunoassay similar in principal to the hCG pregnancy test. The device is composed of a strip of nitrocellulose membrane with embedded ‘capture’ lines one for the detection of anti-SARS-Cov-2 antibodies and a control line to demonstrate the device has worked.
Gold nanoparticles conjugated to a SARS CoV-2-antigen are embedded in the conjugation pad. The patient’s blood or serum sample is deposited at one end of the membrane, and the sample drawn across the entire strip by capillary action. As the sample passes through the conjugation pad, the gold-conjugated SARS-CoV-2 antigen attaches to any reactive antibodies (IgG or IgM) present in the sample.
The sample continues to flow into the membrane until a line of immobilized capture anti-human antibody traps these targeting anti-SARS antigen IgG or IgM molecules in place. Typically, the trapped gold nanoparticles are visualized as a clear red line.
Lateral flow tests are extremely quick (10-20 mins) and require very little technical expertise to interpret the result (Fig. 2). However, the sensitivity, specificity and accuracy of these tests vary widely based on numerous factors, but mainly by the quality of the viral antigen used to detect a signal.
These devices should be considered qualitative rather than quantitative and are principally used for Point of Care testing.
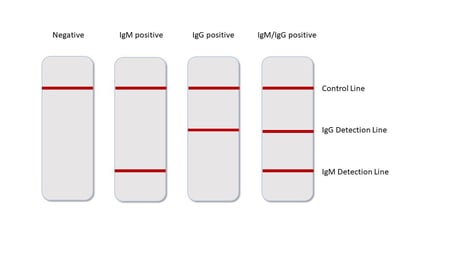
Fig. 2. The readout is simple and clear. The appearance of lines for IgG or IgM or both indicate a positive test result- indicating the patient has been exposed to SARS-CoV-2.
2. ELISA serological testing
The second common serological testing platform is the ELISA. This is not a Point of Care test solution but can provide more sensitivity in testing since the test sample volumes are generally a lot larger than for lateral flow devices, and the signal to noise ratio is higher.
The ELISA platform relies on the absorbance of the testing SARS-CoV-antigen onto the surface of a 96 well plate. Patient serum is added to the wells and any anti-viral antibodies present in the sample will be captured onto the plate.
These captured antibodies are then detected using secondary antibodies targeting human immunoglobulins that are conjugated with the enzyme horseradish peroxidase which converts a substrate 3,3’5,5’ tetramethylbenidine to a colored product that can be read on a plate reader.
This process is scalable, enabling large numbers of clinical specimens to be tested per day, and is semi-quantitative meaning it can provide information on the level of the IgG or IgM immune response to the SARS-Cov-2 antigen.
However, the data needs technical interpretation, and as with the lateral flow devices, the quality of ELISA test is highly dependent on the quality of the viral antigen used on the plate.
How are serological tests validated?
Before any serological tests are used, they need to be validated in order to demonstrate that they are performing to required specifications, which at present has been challenging. Antibody tests for SARS-CoV-2 received Emergency Use Authorization (EUA) from the FDA on 16th March 2020.
This opened the door to the use of unapproved medical products for use in this emergency. Since no antibody tests had been validated at that time, the FDA stated that it “does not expect that an antibody test can be shown to definitively diagnose or exclude SARS-CoV-2 infection.” The recommendations continued to state that healthcare providers should:
- Continue using serological (antibody) tests as appropriate and be aware of their limitations.
- Do not use serological (antibody) tests as the sole basis to diagnose COVID-19 but instead as information about whether a person may have been exposed.
- Be aware that not all marketed serological tests have been evaluated by the FDA. The FDA website lists authorized tests and tests offered under FDA COVID-19 diagnostic policy guidance.
Following the initial positioning by the FDA, there was confusion over exactly how labs were supposed to validate these assays. For Serological Testing, the Clinical Laboratory Improvement Amendments (CLIA) guidelines require a minimum of 25 patient samples, run over several days with different analysts. But access to ‘known-positives’ from individuals who had been able to access the very limited RT-PCR testing, was a challenge early on.
The quality of serological testing is still an issue Globally, and the EU recently (early April, 2020) declared that none of the tests available to them at that time were valuable. The FDA is now collaborating with the National Institutes of Health and Centers of Disease Control Prevention on validating Serological Tests for SARS-CoV-2.
So, if the quality of the viral antigens employed in these serological tests is so important, what can we do to ensure we are working with the optimal viral antigen materials?
There are a few different factors to consider here. Firstly, what do we need these tests to really measure?
An optimal humoral immune response to SARS-CoV-2 would include the production of Neutralizing Antibodies. These are antibodies that target the parts of the virus that binds to- and unlocks our host cells. These viral regions are also the most effective targets for vaccine development, but when it comes to current serological testing, we fall short of deciphering a general immune response to the virus, versus immunity through the production of Neutralizing Antibodies.
This is largely because the first wave of serological test kits was prepared using rapidly and inexpensively generated viral antigen, that poorly represented the native protein structure, and most likely was not engineered to be specific to SARS-CoV-2. In fact, even now, most of the commercially available test kits provide very limited information about the antigen material they use, and commercially available SARS-CoV-2 antigen material is poorly expressed and purified. Now that more independent researchers have been able to study the SARS-CoV-2 viral genome and identify strain specific antigen regions, the expectation is that the quality of these critical research materials will now rapidly improve.
Structural proteins of SARS-CoV-2
The SARS-CoV-2 coronavirus belongs to the beta-CoV-2 genera in the family of Coronaviridae and is a type of positive-sense single-stranded RNA virus. Like other types of coronavirus, SARS-CoV-2 has four structural proteins: the nucleocapsid protein that holds the RNA genome of the virus; and the Spike (S), Envelope (E) and Membrane (M) proteins that collectively create the viral envelope that forms the virus particle. The homotrimers of the Spike protein forms the key that unlocks the host cell and enables the virus to fuse with the host cell membrane, by binding to the receptor angiotensin converting enzymes 2 (ACE2) (The Economist, March 2020; Hoffman, M. et. al. 2020).
The spike protein
The Spike protein, particularly the region of the Spike protein called the receptor-binding domain (RBD) appears to be the most immunogenic viral protein, and is most likely to give rise to those potent Neutralizing Antibodies, based on previous studies with SARS1 and MERS (Zhu, et. al. 2007). However, the Nucleocapsid protein appears to also be highly immunogenic and has been largely adapted for use in the first round of Lateral Flow and ELISA serological test due to its relative ease of recombinant production.
This is concerning since the nucleoprotein is highly conserved between SARS coronaviruses and has the potential for cross-reactivity that may lead to false-positive serological testing results.
As with many viral proteins, both the Nucleocapsid and Spike proteins of SARS-CoV-2 are both highly glycosylated. The virus uses this coat of glycosylation to help avoid detection by the host immune system. This poses challenges when it comes to producing recombinant viral antigen in the lab for applications in serological test development, since it is not easily replicated in vitro.
Critical factors in designing and producing SARS-CoV-2 antigens for serological testing include the selection of viral protein regions that are highly specific for SARS-CoV-2, and finding a means of expressing these recombinant proteins in such a way as to make then as close to the native protein as possible. To this end, recombinant expression in human cell lines can help to ensure protein folding and post translational modifications as close to the native protein as possible.
Protein folding influences the presentation of the protein antigen regions and can significantly impact the performance of the serology test with respect to accuracy, specificity and sensitivity.
Second-generation serological tests
The expectation is that second generation serological tests will employ specifically engineered SARS-CoV-2 antigens that are derived from immunogenic regions that are unique to this virus, and recombinantly produced to ensure they closely resemble the native proteins. Serological distinction of an immune response versus immunity is some way off. However, by combining the use of various viral antigens into a serological testing panel, we may be able to obtain a clearer readout of an individual’s specific immune response to SARS-CoV-2 (Long, Q-X. et. al. 2020; Song, H.C., et. al. 2020). This will also be a valuable tool in the wider-spread adoption of therapeutics such as convalescent serum treatments and for the development of desperately needed vaccines.
Ref.
Wu, L-P. et. al (2007) Duration of Antibody Responses after Severe Acute Respiratory Syndrome. Emerg. Infect. Dis 13(10): 1562-1564.
Hoffman M, Kliene-Weber H, Krüger N, Herrler T, Erichsen S, Schiergens TS, et al. (April 2020). "SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor". Cell. 181 (2): 271–280
Anatomy of a Killer: Understanding SARS-CoV-2 and the drugs that might lessen its power". The Economist. 12 March 2020. Archived from the original on 14 March 2020. Retrieved 14 March 2020
Zhu, Z., et al.
Potent cross-reactive neutralization of SARS coronavirus isolates by human monoclonal antibodies
Proc. Natl. Acad. Sci. U. S. A., 104 (2007), pp. 12123-12128
Long, Q-X., et. al. (2020) Antibody responses to SARS-CoV-2 in patients with COVID-19. Nature Medicine. https://doi.org/10.1038/s41591-020-0897-1
Song, H. C., et. al. (2020) Synthesis and Characterization of a Native, Oligomeric form of Recombinant Severe Acute Respiratory Syndrome Coronavirus Spike Protein. J. Virology 78(19) 10328-10335.
 |
Authored by: Dr. Julie Bick |
Dr Julie Bick is a medicinal biochemist who has spent close to 7 years with FlowMetric Life Sciences. After receiving her doctorate in Biochemistry at Southampton University in the UK, she began her career as Associate Professor at Rutgers University, NJ, before moving to the west coast to perform biomedical research with Syngenta and Novartis at the Tory Mesa Research Institute in San Diego. Dr. Bick specializes in biomedical engineering of cells and proteins in order to provide innovative therapeutic and diagnostic solutions. She brings to FlowMetric a clinical expertise across a wide range of therapeutic areas from autoimmunity to oncology and chronic inflammatory conditions, acquired over 25 years of research experience in academic, biotechnology and pharmaceutical laboratories. In leading FlowMetric Life Sciences’ innovation initiatives, Dr. Bick has been collaborating with BurstIQ to implement Block Chain solutions into the company’s Contract Research Organization division, with a focus on enhanced big data analytics and process control solutions in the regulated clinical environment. Dr. Bick is committed to working with local Community Colleges to support STEM programs for the next generation of scientists.
















